Advances in Improving Outcomes in Patients With Severe Vernal Keratoconjunctivitis (CME Monograph)
Activity Description and Purpose
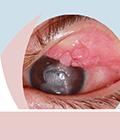
Vernal keratoconjunctivitis (VKC) is a rare form of chronic recurrent ocular allergy that primarily affects young males. The signs and symptoms of the condition can overlap with those of other allergic ocular conditions. A delayed diagnosis can lead to missed treatment opportunities and deleterious long-term consequences, including vision loss. Optimal treatment strategies include the use of traditional approaches and the integration of a newly approved treatment. The purpose of this educational activity is to review the epidemiology of VKC and to provide an overview of current diagnostic and management approaches. The desired results of this activity are for clinicians to better identify and treat patients with VKC.
Target Audience
This educational activity is intended for ophthalmologists.
Learning Objectives
After completing this activity, participants will be better able to:
- Describe clinical trial data for a new treatment for vernal keratoconjunctivitis
- Apply evidence-based treatment strategies for a variety of patients with vernal keratoconjunctivitis
Faculty
 | Asim Ali, MD, FRCSC (Chair) |
 | Simon Fung, MD |
Disclosure Policy
MedEdicus adheres to the ACCME’s Standards for Integrity and Independence in Accredited Continuing Education. Any individuals in a position to control the content of a CME activity, including faculty, planners, reviewers, or others, are required to disclose all financial relationships with ineligible entities (commercial interests). All relevant conflicts of interest have been identified and mitigated by MedEdicus prior to the commencement of the activity.
Faculty
Asim Ali, MD, is a consultant for Santen Inc; and is a contracted researcher for Novartis Pharmaceuticals Corporation.
Simon Fung, MD, is a consultant for Daiichi Sankyo Company, Limited*, Dompé US, Inc, and Santen Inc; and is a contracted researcher for Dompé US, Inc*.
* The financial relationship existed during the past 24 months but has now ended.
Peer Reviewer
This activity was peer reviewed. The peer reviewer has no relevant commercial relationships to disclose.
Planners, Managers, and Writers
MedEdicus planners, managers, and writers have no relevant commercial relationships to disclose.
Accreditation Statement
 MedEdicus LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
MedEdicus LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Credit Designation Statement
MedEdicus LLC designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure of Commercial Support
This continuing medical education activity is supported through an educational grant from Santen Inc.
Off-Label Discussion
This educational activity may include discussion of unlabeled and/or investigational uses of drugs and devices. Please refer to the official prescribing information for each drug or device discussed in this activity for approved dosing, indications, and warnings.
Provider Contact Information
For questions about this educational activity, please contact MedEdicus LLC at [email protected].
Disclaimer
The views and opinions expressed in this educational activity are those of the faculty and do not necessarily represent the views of MedEdicus LLC or Santen Inc.
This CME activity is copyrighted to MedEdicus LLC ©2023. All rights reserved. 264
Available Credit
- 0.50 AMA PRA Category 1 Credit™
- 0.50 Participation

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward