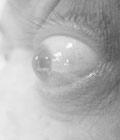
Rethinking Thyroid Eye Disease: Multidisciplinary Approaches to Diagnosis and Treatment (September 30 CE/CME Webinar)
Monday, September 30, 2024
5:00 pm – 6:00 pm PT | 6:00 pm – 7:00 pm MT
7:00 pm – 8:00 pm CT | 8:00 pm – 9:00 pm ET
Activity Description and Purpose
This educational activity aims to provide clinicians with advanced knowledge and competence in identifying the relationship between thyroid eye disease (TED) and comorbid ocular conditions, with a focus on dry eye disease (DED) and ocular surface disease (OSD). Topics covered in this activity include differential diagnoses for TED in patients with proptosis, how DED/OSD overlap, and data on medications used to treat TED. This educational activity integrates didactic content with clinical case studies and panel discussions with experts to reinforce best practices in the management of TED in patients with DED and OSD. At the completion of this activity, clinicians will understand how to effectively position themselves within the multidisciplinary team to achieve positive patient outcomes in patients with TED and DED and OSD. The desired results of this activity are to increase appropriate treatment of TED in practice and to foster competent interdisciplinary collaboration to ensure the best possible outcomes for patients with TED.
Target Audience
This educational activity is intended for ophthalmologists and optometrists.
Learning Objectives
After completing this activity, participants will be better able to:
- Identify the relationship between thyroid eye disease and comorbid ocular conditions
- Review clinical data on treatments for thyroid eye disease that will help inform referral practices
- Apply best practices to cases involving comorbid ocular conditions
Faculty
 | Kenneth A. Beckman, MD, FACS (Chair) |
 | Paul Karpecki, OD, FAAO Director, Cornea and External Disease Kentucky Eye Institute Lexington, Kentucky Associate Professor University of Pikeville School of Optometry Pikeville, Kentucky |
 | Wendy W. Lee, MD, MS Professor of Clinical Ophthalmology and Dermatology Oculofacial Plastic & Reconstructive Surgery, Orbit, and Oncology Bascom Palmer Eye Institute University of Miami Miller School of Medicine Miami, Florida |
Disclosure Policy
MedEdicus adheres to the ACCME’s Standards for Integrity and Independence in Accredited Continuing Education. Any individuals in a position to control the content of a CME activity, including faculty, planners, reviewers, or others, are required to disclose all financial relationships with ineligible entities (commercial interests). All relevant conflicts of interest have been identified and mitigated by MedEdicus prior to the commencement of the activity.
Faculty
Kenneth A. Beckman, MD, is a consultant for AbbVie Inc, Alcon, Amgen, Bausch & Lomb Incorporated, Bruder Healthcare, BVI, Dompé US, Inc, Glaukos Corporation, Invirsa Ic, Johnson & Johnson Vision Care, Inc*, Novartis Pharmaceuticals Corporation*, Ocular Science, Shenyang Sinqi Pharmaceutical Co, Ltd, Sun Pharmaceutical Industries, Inc*, Thea Pharma Inc, Trukera Medical, Verséa Health, Inc, Viatris Inc, Visus Therapeutics, and Yuyu Pharma, Inc; is a contracted researcher for Glaukos Corporation; and has stocks or stock options in RxSIGHT*.
Paul Karpecki, OD, is a consultant for AbbVie Inc, AdOM Optical Technologies Ltd, AesculaTech, AI Optics, Alcon, Aldeyra Therapeutics, Ametek, Inc, Amgen Inc*, Apellis Pharmaceuticals, Aramis, Astellas Pharma Inc, Atlas Medical GmbH, Aurion Biotechnologies, Avellino*, Azura Ophthalmics, Barti, Bausch & Lomb Incorporated, Bio-Tissue, Bruno Vision, Dompé US, Inc, Essilor of America, Inc, Eyenovia*, Harrow, Inc, Hilco Vision, iCare USA Inc, Kiora Pharmaceuticals, Inc*, Konan Medical USA, Inc*, Lentechs, LKC Technologies, Inc, Mitotech Ltd*, Neurolens, Novaliq GmbH*, Novartis Pharmaceuticals Corporation*, OASIS Medical, OCULUS, Inc, OCuSOFT Inc, OcuTerra Therapeutics*, Orasis Pharmaceuticals, Reichert, Inc, Rendia, Inc, RVL Pharmaceuticals, Inc*, RxSIGHT, Samsara, ScienceBased Health, Scope, Sentiss Pharma Pvt Ltd, Sight Sciences, Sun Pharmaceutical Industries, Inc, Surface Pharmaceuticals Inc, Sydnexis Inc, Tarsus Pharmaceuticals, Inc, Thea Pharmaceuticals Limited*, Topcon Medical Systems, Inc, Trukera, Twenty/Twenty Therapeutics*, Vial, Viatris Inc, Visant Medical, Inc*, Visus Therapeutics*, Vital Tears LLC, WebMD, and Zeiss; is a contracted researcher for Amber and Lumibird; is an advisory board member of Silk Technologies Ltd*; has stock options that have not been exercised in BlephEx, Eyedaptic Inc, Eyedetec Medical, Healthe, Hue.AI, iVeena Delivery Systems, Ocuphire Pharma, Omega Ophthalmics, Ophthalmic Resources Partners, Silk Technologies Ltd*, TearClear, and Visant Medical, Inc*; and has received honorarium from Mallinckrodt.
Wendy W. Lee, MD, MS, is a consultant for AbbVie Inc, Amgen Inc, Novabay Pharmaceuticals, Inc, Revance Therapeutics, Inc, RVL Pharmaceuticals, Inc*, and Tarsus Pharmaceuticals, Inc; and is a contracted researcher for Viridian Therapeutics, Inc.
Peer Reviewer
This activity was peer reviewed. The peer reviewer has no relevant commercial relationships to disclose.
Planners, Managers, and Writers
MedEdicus planners, managers, and writers have no relevant commercial relationships to disclose.
* The financial relationship existed during the past 24 months but has now ended.
Accreditation Statement
 MedEdicus LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
MedEdicus LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Credit Designation Statement
MedEdicus designates this live activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
![]() COPE approved for 1.0 CE credit for optometrists.
COPE approved for 1.0 CE credit for optometrists.
COPE Course ID: 92831-SD (Synchronous Virtual)
COPE Course Category: Systemic/Ocular Disease
Administrator: ![]()
This activity, COPE Activity Number 129019, is accredited by COPE for continuing education for optometrists.
Disclosure of Commercial Support
MedEdicus LLC has received commercial support from Amgen Inc for this activity in the form of an unrestricted educational grant.
The content of this ACCME-/COPE-accredited CME/CE activity was planned and prepared independently by MedEdicus LLC without input from members of an ineligible company.
Off-Label Discussion
This educational activity may include discussion of unlabeled and/or investigational uses of drugs and devices. Please refer to the official prescribing information for each drug or device discussed in this activity for approved dosing, indications, and warnings.
Provider Contact Information
For questions about this educational activity, please contact MedEdicus LLC at [email protected].
Disclaimer
The views and opinions expressed in this educational activity are those of the faculty and do not necessarily represent the views of MedEdicus LLC or Amgen Inc.
©2024 MedEdicus LLC. 323.2a
Available Credit
- 1.00 AMA PRA Category 1 Credit™
- 1.00 COPE
- 1.00 Participation

 Facebook
Facebook X
X LinkedIn
LinkedIn Forward
Forward